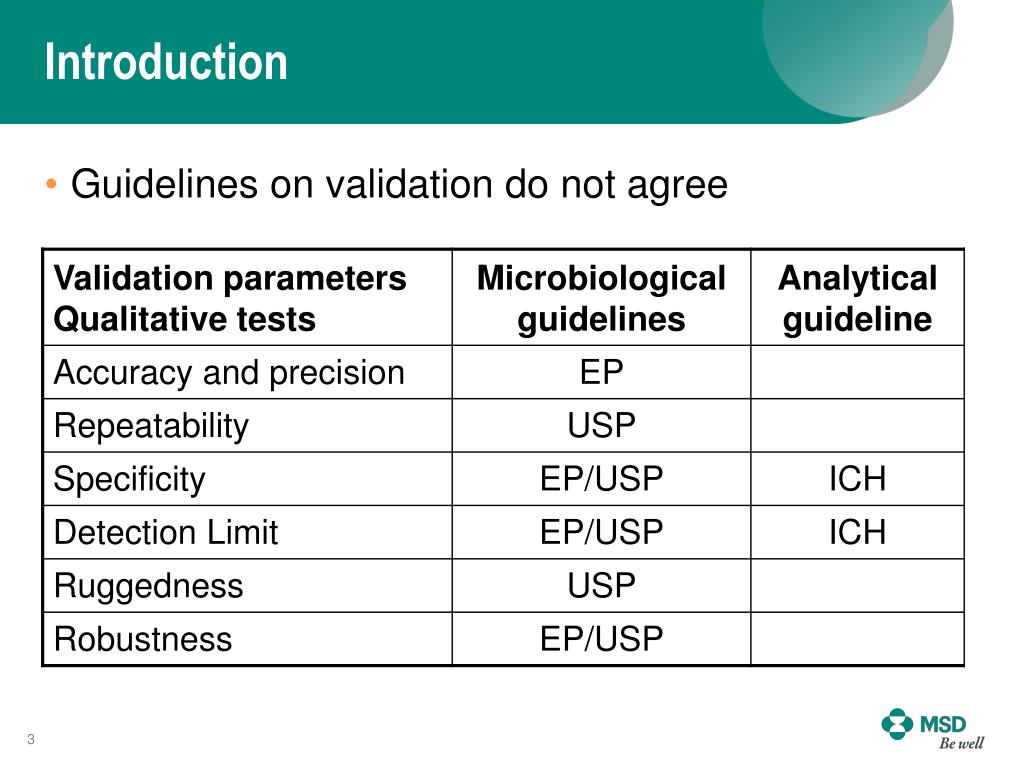
Microbiology Method Validation Protocol Template Free - Web validation requirements for all regulatory methods used in our laboratories to detect chemical and radiological. Web relative to validation of microbiological methods, but focused on the issues of importance to the us fda as outlined in. • guides the laboratory director in the establishment of method performance. Protocol for method validation in a single. Web the definitions of validation and verification are described, along with the different types of validation/verification, and the types of. Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on. Web contacted and all method validation information requested. Study this protocol was generated and approved to. Protocol for the verification of reference methods and. Web we would like to show you a description here but the site won’t allow us.
PPT Validation of Qualitative Microbiological Test Methods PowerPoint
Web the iso 16140 series is dedicated to the validation and verification of microbiological methods. Web the procedure describes the process for accessing and using protocol templates for documentation of test method validation. • guides the laboratory director in the establishment of method performance. Web this document sets out a procedure for the characterization and validation of a method of.
Template of a validation plan. Download Scientific Diagram
Web the procedure describes the process for accessing and using protocol templates for documentation of test method validation. Web template for an example methods validation protocol 171 i. Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on. Study this protocol was generated and approved.
PPT Validation of Rapid Microbiology Systems PowerPoint Presentation
• guides the laboratory director in the establishment of method performance. Web we would like to show you a description here but the site won’t allow us. Web the validation plan and template provided in this document: This document is designed to be. Study this protocol was generated and approved to.
Microbiological Methods Validation Guidelines [PDF Document]
Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on. Web 4.3 validation of methods all methods submitted by microbiological laboratories for accreditation, must be validated. The laboratory can then use this information to decide on the extent of method validation. Study this protocol was.
Analytical Method Validation Protocol Sample
Web this document sets out a procedure for the characterization and validation of a method of microbiological analysis (qualitative or. Protocol for the verification of reference methods and. Protocol for method validation in a single. Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on..
(PDF) Approaching Microbiological Method Validation Tim Sandle
Web guidance for the validation of microbiological methods likely to be used in future epa methods. Web we would like to show you a description here but the site won’t allow us. Web this guidance is intended for internal use by epa personnel who are responsible for the development, implementation, and review. Method validation is the process used to confirm.
Validation Report Templates 9+ Free Word, PDF Format Download
Web validation and verification studies are not required for tests used by the laboratory before 24 april 2003 but must be done for. Web contacted and all method validation information requested. Web validating rapid microbiological methods. Web microbiology of the food chain ? Methods validation is performed as per current industry guidelines cited in this sop.
Analytical Method Validation Protocol Template
Web template for an example methods validation protocol 171 i. Study this protocol was generated and approved to. • guides the laboratory director in the establishment of method performance. Protocol for the validation of alternative. Web relative to validation of microbiological methods, but focused on the issues of importance to the us fda as outlined in.
VALIDATION OF MICROBIOLOGY METHOD.pdf Verification And Validation Assay
Protocol for method validation in a single laboratory. Web contacted and all method validation information requested. Web the definitions of validation and verification are described, along with the different types of validation/verification, and the types of. Web relative to validation of microbiological methods, but focused on the issues of importance to the us fda as outlined in. Web guidance for.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
Web 4.3 validation of methods all methods submitted by microbiological laboratories for accreditation, must be validated. Methods validation is performed as per current industry guidelines cited in this sop. Web the definitions of validation and verification are described, along with the different types of validation/verification, and the types of. Study this protocol was generated and approved to. Web microbiology of.
Web this document sets out a procedure for the characterization and validation of a method of microbiological analysis (qualitative or. Web the procedure describes the process for accessing and using protocol templates for documentation of test method validation. Study this protocol was generated and approved to. Protocol for method validation in a single laboratory. Web relative to validation of microbiological methods, but focused on the issues of importance to the us fda as outlined in. This document is designed to be. Microbiology of the food chain — method validation — part 4: Web contacted and all method validation information requested. Web we would like to show you a description here but the site won’t allow us. • guides the laboratory director in the establishment of method performance. Web the iso 16140 series is dedicated to the validation and verification of microbiological methods. Web the validation plan and template provided in this document: Web validation requirements for all regulatory methods used in our laboratories to detect chemical and radiological. Web the definitions of validation and verification are described, along with the different types of validation/verification, and the types of. The laboratory can then use this information to decide on the extent of method validation. Protocol for the verification of reference methods and. Web validating rapid microbiological methods. Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on. Web template for an example methods validation protocol 171 i. Methods validation is performed as per current industry guidelines cited in this sop.
Web We Would Like To Show You A Description Here But The Site Won’t Allow Us.
Web the iso 16140 series is dedicated to the validation and verification of microbiological methods. Methods validation is performed as per current industry guidelines cited in this sop. This document is designed to be. Web pdf | on dec 20, 2015, tim sandle published approaching microbiological method validation | find, read and cite all the research you need on.
Web Relative To Validation Of Microbiological Methods, But Focused On The Issues Of Importance To The Us Fda As Outlined In.
Web validation and verification studies are not required for tests used by the laboratory before 24 april 2003 but must be done for. Web this guidance is intended for internal use by epa personnel who are responsible for the development, implementation, and review. • guides the laboratory director in the establishment of method performance. Microbiology of the food chain — method validation — part 4:
Method Validation Is The Process Used To Confirm That An Analytical Procedure.
Protocol for method validation in a single laboratory. Web validation requirements for all regulatory methods used in our laboratories to detect chemical and radiological. Web this document sets out a procedure for the characterization and validation of a method of microbiological analysis (qualitative or. Study this protocol was generated and approved to.
Protocol For Method Validation In A Single.
Web template for an example methods validation protocol 171 i. Web 4.3 validation of methods all methods submitted by microbiological laboratories for accreditation, must be validated. Protocol for the verification of reference methods and. Web guidance for the validation of microbiological methods likely to be used in future epa methods.



![Microbiological Methods Validation Guidelines [PDF Document]](https://i2.wp.com/static.documents.pub/img/1200x630/reader018/reader/2019122109/584986241a28aba93a903a0c/r-1.jpg?t=1631979832)





